250.102(A) Grounded Conductor, Bonding Conductors, and Jumpers.
Section 250.102(A) makes it clear that bonding jumpers shall be of copper, aluminum, copper-clad aluminum, or other corrosion-resistant material. Resistance to corrosion is critical to ensure future integrity of a bonding jumper.
Both copper and aluminum are susceptible to corrosion and there are many misconceptions regarding the corrosion resistance of each material. Between copper and aluminum, copper is the most cathodic, or noble, and the effects of galvanic action is typically less in copper over aluminum. As a result, the general opinion of the electrical industry is that copper is corrosion resistant in almost any environment and aluminum is regarded as non-corrosion-resistant. However, placing copper in contact with more noble metals such as nickel or titanium would result in the copper corroding just as readily as aluminum. Installation conditions and other environmental factors play a big role in corrosion resistance.
Galvanic action leading to corrosion can occur when two dissimilar metals are in contact with one another in an electrically conductive fluid (electrolyte).
For this to occur, the following conditions must exist:
- There must two dissimilar metals in contact with one another; an anode (the less noble metal that will corrode) and a cathode (the more noble metal that will be protected through the corrosion process).
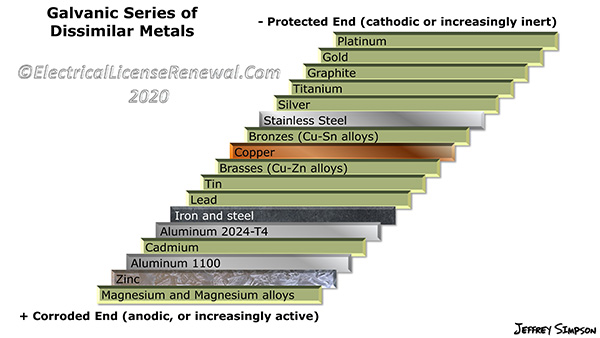
- There must be different corrosion potential between the anode and the cathode (see illustration).
- There must be an electrolyte (electrically conductive fluid) in contact with the two metals.
During the corrosion process, the less noble metal (anode) deteriorates at an accelerated rate and deposits portions of itself onto the cathode as the anode tries to return to its original state before it was refined (ore).
Aluminum and galvanized steel for example, are reasonably compatible with one another (see illustration) unless a corrosive agent is introduced which will accelerate the corrosion process. The closer the dissimilar metals are together on the dissimilar metals chart, the slower the corrosion process will be when the two dissimilar metals are in contact and an electrolyte is introduced. Likewise, the further the two dissimilar metals are from one another on the dissimilar metals chart, the faster the corrosion process will be when the dissimilar metals are in contact and an electrolyte is introduced.
Relative surface area of the two dissimilar metals is also a factor. The rate of corrosion will depend on which of the two dissimilar metals has the larger surface area and the nobility of the two metals. Example: a small stainless-steel (more noble) screw or rivet driven through a large galvanized steel (less noble) surface will last significantly longer when introduced to an electrolyte than a small galvanized steel screw or rivet driven into a large stainless-steel surface.
Below is a preview of the NEC®. See the actual NEC® text at NFPA.ORG for the complete code section. Once there, click on their link to free access to the 2020 NEC® edition of NFPA 70.
2020 Code Language:
250.102 Grounded Conductor, Bonding Conductors, and Jumpers.
(A) Material. Bonding jumpers shall be of copper, aluminum, copper-clad aluminum, or other corrosion-resistant material. A bonding jumper shall be a wire, bus, screw, or similar suitable conductor.